


Biolocity is an incubator at Emory University & Georgia Institute of Technology focused on advancing life science technologies to the market. In October 2019, Biolocity was selected to join the BARDA DRIVe Accelerator Network. In this role, we have been working with BARDA to identify companies and innovative technologies that help address 21st-century health security threats, with a recent focus on COVID-19. In response to the Coronavirus public health emergency, we received numerous questions from members of our ecosystem and we saw an opportunity to share BARDA DRIVe’s perspective as well as their funding and partnership opportunities.


Biolocity recently sat down with our partners at BARDA DRIVe to learn more about BARDA-specific response to COVID-19 and how our ecosystem can help address this public health emergency.
Dr. Sandeep Patel, DRIVe Director, offered his thoughts.
|
 Dr. Sandeep Patel DRIVe Director |
Tell us a little bit about BARDA and DRIVe. What makes DRIVe unique within BARDA?
The Biomedical Advanced Research and Development Authority or BARDA, as we are known, is part of the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR). BARDA invests in the innovation, advanced research and development, acquisition, and manufacturing of medical countermeasures – vaccines, drugs, therapeutics, diagnostic tools, and devices needed to combat health security threats, including pandemics like COVID-19, and save lives. Our development goal is always regulatory approval, licensure, or clearance.
Within BARDA, DRIVe, the Division of Research, Innovation and Ventures, accelerates the development and availability of transformative technologies and approaches, particularly to challenges that span all health security threats, like early detection of infection and personal notification to act, control, and treat infections, or even sepsis. We have some unique funding capabilities and responsive timelines that increase the agility of BARDA to respond to rapidly emerging threats with innovative technologies. We partner with companies and teams offering solutions to a broad range of national health security threats. DRIVe partnering approaches vary depending on the project. BARDA has two currently open solicitations, our EZ-BAA for awards under $749K and our BARDA BAA for more advanced projects.
What is the role of DRIVe in the BARDA response to COVID-19?
DRIVe has been 100% focused on developing and delivering needed capabilities to address COVID-19. We temporarily shifted our usual DRIVe areas of interest in order to focus our EZ BAA intensively on COVID-19 medical countermeasure development at this time. Not surprisingly, many of the technologies that we were already supporting in our DRIVe portfolio to Solve Sepsis or identify early infection were nicely suited to also address SARS-CoV-2 and COVID-19. To add to these efforts, we are soliciting proposals and quickly making awards for the development of SARS-CoV-2 diagnostic tests, vaccines, and advanced pharmaceutical manufacturing technologies. In the three months since we broadened our aperture and published the EZ BAA COVID-19 special instructions, we’ve received and processed more applications than we did over an entire year at a normal operating pace. Incredibly, we’ve been able to maintain our rapid review turnaround cycles, awarding the first COVID-19 diagnostics contracts in as few as twelve days.
How can innovators keep apprised of BARDA’s areas of interest related to COVID-19?
Follow us @BARDA on LinkedIn and check out our evolving portfolio on medicalcountermeasures.gov. To support ongoing U.S. government COVID-19 medical countermeasure development efforts, BARDA is pleased to offer our EZ-BAA and BARDA BAA. These open solicitations seek abstract or white paper submissions for select COVID-19 medical countermeasures. For up-to-date information on the BARDA COVID-19-focused areas of interest, you can visit our COVID-19 EZ BAA website or the BARDA website, which also describes a number of other ways to engage with us one-on-one. Interested parties can also request a CoronaWatch Meeting. This is an interagency, single point of entry portal for vaccines, therapeutics, diagnostic assays, or other products and services related to COVID-19. These market research submissions allow ASPR, BARDA, NIH, NIAID, FDA, CDC, and DoD colleagues to also become aware of potential COVID-19 projects in development.
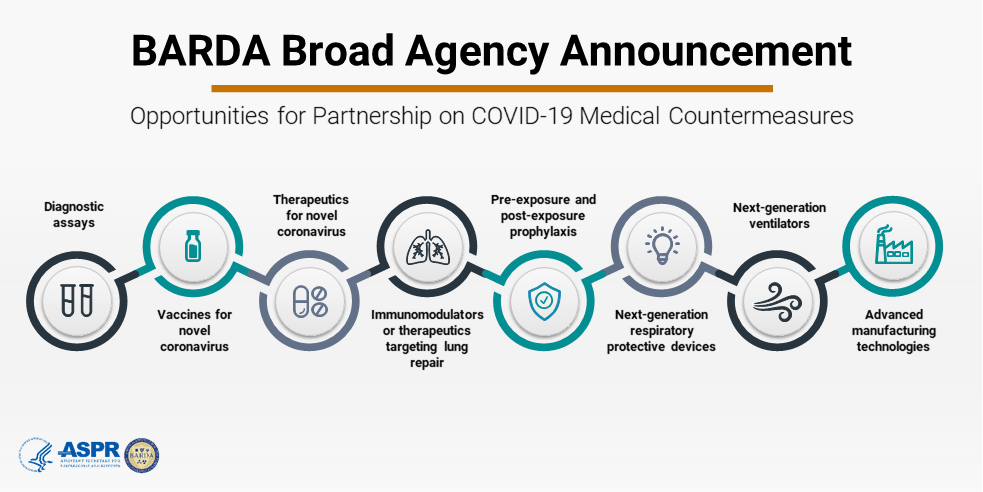
With the range of Federal funding resources in response to COVID-19, how does BARDA’s interest vary from or complement others like the NIH, DoD, and FDA?
We are working closely with our HHS agencies—NIH, FDA, and CDC—and other federal partners to leverage our efforts when able and in this way build the most robust portfolio of products and technologies to address our shared areas of interest. HHS, DoD, Homeland Security, and others have pandemic preparedness programs that collaborate with each other, from early research to advanced development, procurement, and deployment. During a public health emergency response, it is vital that we are in constant communication with our federal partners along the acquisition spectrum. BARDA, NIH, CDC, and the FDA complement each other across the research, development, procurement, and deployment of medical countermeasures. BARDA typically focuses on the advanced research and development of medical countermeasures, partnering with industry to provide funding and expertise to pursue FDA approval, clearance or authorization, and ultimately make available safe and effective medical countermeasures to protect Americans. For the COVID-19 response, each agency is working more closely and coordinated on medical countermeasure development than we have ever seen in the past. To be successful, it is critical that we are all coordinated and hyper-focused on accelerating the development of life-saving medicines, vaccines, and diagnostics to halt this pandemic.
What is the best way for innovators to engage BARDA if they have a solution that will impact the response to COVID-19?
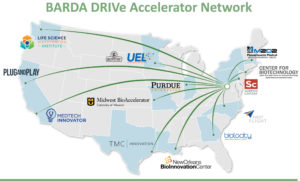 We encourage them to reach out to our national network of BARDA DRIVe Accelerator Network members, including Biolocity, to receive a quick consultation on the fit and area of interest of your technology with current BARDA partnership opportunities. Through our accelerator network partnerships, DRIVe is decentralizing innovation across the country with regional accelerators, selected through a competitive process, to identify promising innovation and provide wraparound technical and business development support services.
We encourage them to reach out to our national network of BARDA DRIVe Accelerator Network members, including Biolocity, to receive a quick consultation on the fit and area of interest of your technology with current BARDA partnership opportunities. Through our accelerator network partnerships, DRIVe is decentralizing innovation across the country with regional accelerators, selected through a competitive process, to identify promising innovation and provide wraparound technical and business development support services.
Anyone developing a technology is encouraged to submit a request with a short description of their technology to the intergovernmental COVID-19 CoronaWatch program at medicalcountermeasures.gov. Submissions to this portal are reviewed by BARDA and our federal government partners, relevant technologies that meet an agency’s needs are invited to have a market research meeting. As needs emerge and change in response to the COVID-19 pandemic agencies have the ability to query previous submissions. The interagency COVID-19 CoronaWatch program was launched specifically to help companies streamline the process of preparing their proposals. Before companies would have to reach out separately to multiple agencies however, with this portal they only have to submit one request which is accessible to all interested U.S. government agencies which saves them valuable time and resources.
How can applicants tell if their technology is a better fit for the BARDA EZ-BAA funding mechanism or the regular BARDA BAA?
A great start would be to consult with one of the accelerators in our BARDA DRIVe Accelerator Network. These accelerators were selected through a competitive process to work with us as extensions of ourselves, scouting and coaching potential applicants for BARDA’s areas of interest. We work with them regularly and they are up to speed on the latest information about our EZ BAA and BARDA BAA, and COVID-19 focus areas.
The best way is to review the EZ-BAA and BARDA BAA solicitations themselves, where companies can find in-depth information applicable to their technology. Typically, technologies submitted to the BAA are more advanced in technological readiness than technologies submitted to the EZ-BAA.
What are some of the common traits of successful awardees for both the EZ-BAA and BAA?
BARDA has worked successfully with companies of all sizes and scopes, from small entrepreneurial groups to biotech groups to large multinational organizations. So, there really is not a set of “common” traits for awardees, it varies in terms of what companies are offering and the technological readiness of their products and technologies. Both of these BAAs describe a wide variety of our product interest areas aimed at getting the COVID-19 response capacity developed and deployed as quickly as possible but also looking toward long-term solutions to this problem that will be around for some time and strengthen our everyday health system. The criteria are explained in each BAA, but again our Accelerator Network can help and you can always reach out to us too, we are happy to answer questions.
How does BARDA typically engage with awardees? How has this changed as part of the COVID-19 response?
The process itself hasn’t changed. BARDA works side by side with its industry and start-up partners. BARDA is not just a funding organization, we provide project support, technical expertise, and oversight throughout the partnership. BARDA partners can access a deep pool of product development, regulatory, manufacturing as well as non-clinical and clinical subject matter experts throughout the partnership. BARDA program teams meet regularly with partners to ensure projects are running smoothly and issues are being addressed as quickly as possible.
Anything else we should know about the BARDA & DRIVe COVID-19 opportunities for partnership?
Yes: please follow us on social media (@BARDA) for all the latest on the COVID-19 response and our other mission areas. You can also visit our COVID-19 Portfolio Page to see what companies we have partnered with to end the pandemic. And one more thing – we are hiring! You can see recent postings on BARDA’s Twitter @BARDA and BARDA LinkedIn pages as well as on USAJOBS.gov.
About Biolocity
Biolocity is a philanthropic, multi-institutional network supporting university medical technology commercialization in the southeast. Through a combination of investment, project management, consulting, and educational programming, Biolocity provides early-stage, patient impacting technologies with the resources needed to reach critical commercialization milestones.

Contact
Christina Wessels
Program Coordinator, Biolocity
Wallace H. Coulter Department of Biomedical Engineering
Emory University & Georgia Institute of Technology






You must be logged in to post a comment.